High Dose Vitamin C Autoimmune
Introduction
Vitamin D is a secosteroid and prohormone which can be obtained from food (as either vitamin D2 or D3), but whose main source is endogenous production in the skin. This requires ultraviolet-B radiation (290-315 nm) of at least 18 mJ/cm2 intensity (1), inducing the formation of previtamin D3 from 7-dehydrocholesterol which subsequently converts to vitamin D3 (cholecalciferol) by body heat (2). Upon reaching the blood, vitamin D3 mainly binds to vitamin-D binding protein (DBP) and gets transported to the liver where it is transformed into its storage form calcidiol (25-hydroxyvitamin D3 or 25(OH)D3) through hydroxylation via the vitamin D3 25-hydroxylases CYP2R1, CYP27A1 or CYP27B1, all members of the cytochrome P450 enzyme family (3, 4). 25(OH)D3 is the main laboratory parameter to judge an individual's vitamin D status; concentrations <20 ng/ml (1ng/ml = 2,5 nmol/l) are considered as vitamin D deficiency, and 40-60 ng/ml as ideal (2, 5). 25(OH)D3 is metabolized in various tissues (predominantly the kidneys) to the biologically most active vitamin D hormone calcitriol (1,25-dihydroxyvitamin D or 1,25(OH)2D3) with another hydroxylation at the 1α position by CYP27A1 or CYP27B1 (3, 4). Besides regulating calcium metabolism, 1,25(OH)2D3 has multiple pleiotropic effects, particularly within the immune system, and is increasingly utilized not only within prophylaxis, but also within therapy of various diseases (2, 6, 7). In particular, binding of 1,25(OH)2D3 to the vitamin D receptor (VDR) has been shown to inhibit the differentiation and proliferation of B and T helper (Th) lymphocytes, promoting the shift of an inflammatory to a more tolerant immune status which may explain the protective effects of vitamin D against autoimmune diseases [reviewed in (8)].
More recently, Slominski and colleagues have revealed alternative pathways of vitamin D metabolism mediated by the mitochondrial enzyme CYP11A1 which is able to hydroxylate the side chain of vitamin D2/D3 (9–11). The main product of these reactions is 20-hydroxyvitamin D3 (20(OH)D3), which has a 20-30-fold lower concentration than 25(OH)D3 in human serum (9, 11) and is the initial substrate for the formation of further hydroxy-derivatives such as 20,23(OH)2D3, 17,20,23(OH)3D3 or 20,22(OH)2D3 [reviewed in (12)]. These nonclassical vitamin D metabolites also act as hormones: besides being partial agonists of the VDR, they have high affinity as agonists of the aryl hydrocarbon receptor (AhR) (13) and as inverse agonists of the retinoid-related orphan receptors (ROR) α and γ (14, 15). Notably, CYP11A1 is expressed in immune cells (16) and RORα and RORγ are expressed by inflammatory Th17 cells in which they synergistically regulate differentiation and inflammatory cytokine production (17). Th17-derived cytokines, notably interleukin (IL)17, have been implicated in the etiology of autoimmune disorders such as psoriasis (18) and multiple sclerosis (MS) (19). In addition, single nucleotide polymorphisms (SNPs) of the RORA gene have been associated with MS (20). The binding of 1,25(OH)2D3, 20(OH)D and other vitamin D hydroxy-metabolites to both RORα and RORγ, result in IL17 inhibition (14), thus providing another mechanism distinct from VDR signaling how vitamin D may protect from, or alleviate symptoms of, autoimmune diseases.
For approximately 15 years, patients with autoimmune diseases, particularly MS, have been successfully treated using a high-dose vitamin D protocol. Because this method has been developed by Prof. Dr. Cicero Coimbra in Sao Paolo, Brazil, it is frequently referred to as the "Coimbra protocol"; in Germany it is utilized since 2016. Underlying the Coimbra protocol is the hypothesis of a non-hereditary, but acquired form of vitamin D resistance which this paper is going to examine.
A hallmark of acquired vitamin D resistance, if it exists, would be an elevated parathyroid hormone (PTH) concentration despite 25(OH)D3 levels being in the ideal range and thus indicating sufficient production of 1,25(OH)2D3. One key role of 1,25(OH)2D3 is to enhance intestinal calcium absorption. If ionized calcium concentrations in blood are low, the parathyroid glands release PTH which stimulates calcium release from bones. Furthermore, PTH increases the conversion of 25(OH)D3 into 1,25(OH)2D3 in the kidneys with subsequent release into circulation. PTH also inhibits the tubular reabsorption of phosphate which in turn lowers the amount of water-insoluble calcium-phosphate salts and thus increases ionized calcium concentrations. In this way, PTH constitutes a direct feedback mechanism within the vitamin D system. A physiological 25(OH)D3 level should thereby be able to suppress PTH into the lower third of the reference range. In other words: If 25(OH)D3 levels are high, PTH should be low and vice versa. In patients with autoimmune diseases this negative feedback loop is disturbed. Based on these observations, Prof. Coimbra proposed the hypothesis of a vitamin D resistance.
The Hypothesis of Acquired Vitamin D Resistance
In two intervention studies, Carlberg and colleagues found evidence that different individuals display a different molecular and biochemical response to the same dose of either long-term or single-bolus vitamin D3 supplementation (21). Initially, in the VitDmet study, 71 elderly prediabetic individuals were supplemented with either 0, 1600 or 3200 I.U. vitamin D3 daily over 5 months of Finnish winter (22). The focus of this work was on the effects of vitamin D3 supplementation on mRNA expression of twelve of the vitamin D-regulated genes and several vitamin D-affected laboratory parameters. With the help of these biomarkers, the group was able to show in 2015 that even supposedly adequate high vitamin D3 doses (3200 I.U.) were not able to exert the expected vitamin D-regulatory effects in all subjects. Focusing on the PTH feedback system alone, a total of 25% of the patients showed no adequate response of this laboratory parameter. Regarding all 36 tested parameters, Carlberg et al. could cluster their patients in 24% low responders, 51% mid responders and 25% high responders. In 2017, the group was able to reproduce these findings in the VitDbol study, in which a cohort of healthy Finnish students received a 80,000 I.U. bolus dose of vitamin D3 with similar low responder rates (23).
These data provided an in vivo confirmation that there exists a spectrum of different vitamin D responsiveness, with approximately 25% of a population not responding adequately to conventional vitamin D3 doses. They may require individually varying, but larger doses. Vitamin D resistance, as proposed by Prof. Coimbra and this article, could be conceived as the extreme low-response end of the vitamin D responsiveness spectrum. Accordingly, individuals being vitamin D resistant would require very high doses of vitamin D3 supplementation to achieve an adequate physiological response, such as a reduction of PTH concentrations or the down-regulation of an activated adaptive immune system – the latter effect is important for the treatment of autoimmune diseases as discussed further below.
Indeed, the idea of vitamin D resistance has first been proposed in 1937 by Albright, Butler and Bloomberg based on the observation that in rare cases of rickets in children, very high doses of vitamin D were required to relieve symptoms (24). It has later been shown that resistance to 1,25(OH)2D3 in such children is frequently caused by hereditary VDR defects, resulting in hypocalcemia, secondary hyperparathyroidism, rickets and in about one half of the patients alopecia (25–27). In contrast to these cases of hereditary vitamin D resistance, which is very rare and already diagnosed in childhood, the hypothesis investigated in this paper concerns a non-hereditary, acquired form of vitamin D resistance which promotes the development of autoimmune diseases. As discussed in more detail below, such a form of vitamin D resistance could develop during aging based on an interaction between genetic susceptibility polymorphisms of the vitamin D system and an accumulation of environmental factors additionally impairing the hormonal signaling of the vitamin D-derived hydroxy-metabolites. Acquired vitamin D resistance is therefore more common than hereditary vitamin D resistance according to the frequency of susceptibility polymorphisms and the rising incidence of autoimmune diseases.
Diagnosis of Vitamin D Resistance
In their article "The concept of the personal vitamin D response index", Carlberg and Haq (21) proposed the following: "A screening using a single vitamin D bolus treatment and measurements at days 0 and 2 paired with a simplified protocol of the molecular analysis used in the VitDbol study [… ] may be the easiest way to identify persons with a low vitamin D response index" (21). Theoretically, people with acquired vitamin D resistance should also be found with this protocol as outliers with extremely low response indices. However, the majority of the biomarkers measured in the VitDbol study can only be determined in research laboratories. Nevertheless, PTH provides a valuable and easy-to-measure biomarker for the clinical therapist. As mentioned above 1,25(OH)2D3 and PTH stand in a direct relationship with each other. A high PTH concentration in turn is associated with a higher all-cause-mortality in epidemiological studies (28). Hence, the recommended optimal 25(OH)D3 level of >40ng/ml should correlate physiologically with an optimal PTH concentration. Because reference ranges and measurement units for PTH vary among different laboratories, it could be stated that a 25(OH)D3 measurement >40 ng/ml should be associated with a PTH value in the middle of the lower third of its laboratory-specific reference range. In such a case, high PTH values are indicative for vitamin D resistance, assuming that dietary calcium and phosphate intake are adequate and a differential diagnosis of hyperparathyroidism has been ruled out. For example, if a laboratory's reference range for PTH is 15-65 ng/ml, the middle of the lower third of that range would be 23.3 ng/ml.
Furthermore, the constellation of high serum 1,25(OH)2D3 concentrations with a concurrently physiological 25(OH)D3 concentration is known to occur in hereditary forms of vitamin D resistance or VDR knockout mice (29); it could therefore also be indicative of acquired vitamin D resistance. The 1,25(OH)2D3 concentration is determined on the one hand by the activity of the enzymes CYP2R1, CYP27A1 and CYP27B1 that catalyze production of 1,25(OH)2D3 and on the other hand CYP24A1 which catalyzes 1,25(OH)2D3 degradation. The inactivation of 1,25(OH)2D3 occurs either systemically or locally within the cells of target tissues. Because PTH negatively regulates CYP24A1 and positively regulates the hydroxylases converting 25(OH)D3 to 1,25(OH)2D3, the presence of vitamin D resistance, which results in low intestinal calcium absorption and thus PTH stimulation, would lead to a constant elevation of 1,25(OH)2D3.
In case of a disturbed VDR response, e.g., caused by SNPs, an increased 1,25(OH)2D3 concentration is urgently needed to maintain the integrity of VDR signaling. Therefore, an elevated PTH concentration can result from vitamin D resistance mediated by dysfunctional VDR signaling. It follows that a decreased 25(OH)D3/1,25(OH)2D3 ratio could be perceived as a biomarker for vitamin D resistance. However, because PTH elevation is causally responsible for an increased 1,25(OH)2D3 level, PTH is a clinically sufficient sensitive surrogate biomarker for vitamin D resistance (Figure 1). Especially since, according to our experience, a disturbed 25(OH)D3/1,25(OH)2D3 ratio is perceived as not sufficiently reliable. A reason could be that some cases of vitamin D resistance are based on SNPs in enzymes catalyzing production of 1,25(OH)2D3 such as CYP27B1, as originally proposed by Coimbra and his co-workers (30). In this case, PTH would be elevated, but its action on expression of this dysfunctional enzyme is not sufficient to raise 1,25(OH)2D3 levels.
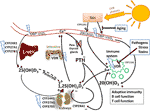
Figure 1 Vitamin D metabolism and the sites of vitamin D resistance acquirement. Vitamin D3 is mainly produced in the skin through solar ultraviolet-B (UVB) radiation and gets transported through blood by binding to vitamin D binding protein (DBP). The liver is the systemic production site of 25(OH)D3 via the enzymes CYP2R1, CYP27A1 or CYP27B1. 25(OH)D3 then gets converted to the active hormone 1,25(OH)2D3 by a second hydroxylation which occurs mainly in the kidneys or in other tissues through paracrine production. 1,25(OH)2D3 binds to the vitamin D receptor (VDR) to promote intestinal calcium (Ca) absorption and other Ca-mobilization pathways (e.g. in bone), so that ionized Ca levels in serum rise. The paraythroid glands sense Ca2+ levels, and secrete parathyroid hormone (PTH). PTH in turn promotes the production of 1,25(OH)2D3, e.g. by inhibiting CYP24A1 which catalyzes the first degradation step of 1,25(OH)2D3. Besides 1,25(OH)2D3, there are other vitamin D3-derived hydroxy-metabolites that exert hormone-like actions. For example, skin also expresses CYP11A1, which promotes the conversion of vitamin D3 into 20(OH)D3, which has non-calcemic biological effects, for example on immune cells. The blue flashes show where polymorphisms can interfere with normal vitamin D metabolism, building the basis for the development of acquired vitamin D resistance. Additional factors that impair vitamin D signaling are highlighted in blue boxes.
Development of Vitamin D Resistance
As we have just illustrated, susceptibility for vitamin D resistance could arise from multiple polymorphisms of genes expressing for different proteins within the vitamin D system: the cytochrome-P450 enzymes (hydroxylases) needed for the conversion of vitamin D2/D3 into the activated hormone form (CYP2R1, CYP27A1 and CYP27B1), the DBP needed for vitamin D transport, the cell-surface receptor megalin-cubilin, which is the membrane receptor for the 1,25(OH)2D3/DBP complex, the VDR itself or the recently discovered other receptors for vitamin D hydroxy derivatives such as RORα and RORγ. Indeed, inactivating polymorphisms of the CYP2R1 (31) or CYP27B1 (32) genes, promoter polymorphisms of CYP24A1 (33) or RORA (20), and SNPs of the VDR gene (34–38) have all been associated with autoimmune diseases. However, as suggested by research on hereditary vitamin D resistance, the VDR is probably the most vulnerable part of the vitamin D metabolic system and constitutes the most likely or at least most potent manifestation of acquired vitamin D resistance.
The Vitamin D Receptor
The VDR is a steroid receptor expressed in almost all cell types of the human body, in particular most immune cells (38). The intracellular distribution of the VDR is usually 75-80% in the nucleus, 15-20% in the cytosol and 3-5% in the plasma membrane (39). Binding of 1,25(OH)2D3 induces translocation of cytosolic VDR into the nucleus in a dose-, time- and temperature-dependent manner (40). In the nucleus, the liganded VDR forms a heterodimer with the unliganded retinoid-X receptor which then binds to DNA and recruits either coactivator or corepressor proteins and other transcription factors to exert gene regulatory functions (39). Due to this property, the VDR is classified as a member of the nuclear receptor family. Other members of this "superfamily" of nuclear receptors are, among others, the thyroid receptor, the estrogen, progesterone and testosterone receptors, the retinoid-X receptor as well as the cortisol receptor (41). The high importance of the VDR is underscored by the great number of genes that it regulates, both as a classical transcription factor and through epigenetic effects (42). For example, Seuter et al. have shown that 1,25(OH)2D3 binding to the VDR modulated the transcription of 1204 genes in the human monocytic cell line THP-1 over 24 hours, mostly by modulating the accessibility to their respective chromatin regions (43). The 46 chromatin strands, which measure up to 5 cm in length and have a diameter of approximately 0.01 mm, are thereby modified in a way that opens up the target genes for reading (44). This epigenetic function of the VDR is exerted in almost all tissue types, which underlines its important role as a regulatory protein well beyond its known function in calcium metabolism (42, 43).
Critically, several VDR SNPs have been revealed that are increasingly associated with multiple autoimmune diseases, either individually or as haplotypes (34–38). For MS, e.g., a recent meta-analysis revealed a significant association with rs731236 (TaqI) polymorphisms when comparing heterozygous (Tt) with homozygous (TT) genotypes (45). The rs731236 GG genotype was also a significant predictor of low vitamin D responsiveness in a supplementation study of 100 Arab women, together with the rs7116978 (CYP2R1 gene) CC genotype (46). This study also provided an overview showing that the rs7116978 G allele and rs7116978 C allele reached high population prevalence of up to 40% or 70%, respectively, depending on ethnicity (46). It is known that certain haplotypes of VDR polymorphisms can influence its mRNA expression levels, stability and protein translation efficiency (47). Furthermore, work of Booth et al. who studied polymorphisms within the context of VDR binding to genes in human monocytes and dendritic cells, suggests that certain polymorphisms predispose to autoimmune diseases by perturbing VDR binding at autoimmune disease risk gene variants (38). In other words, SNPs of the VDR may predispose to acquired vitamin D resistance, which may become manifest during aging and lead to the development of an autoimmune disease, if additional factors accumulate that contribute to an impairment of VDR signaling. These factors will be reviewed in the next subsection.
The Vitamin D Receptor Is Regulated by Multiple Factors
With first evidence for a VDR in 550 million-year old boneless vertebrates, the VDR is a long-established regulatory protein (48). From an evolutionary perspective it appears plausible that such a conserved and seminal protein is subject to multiple influencing factors. We elaborate on this using the example of the glucocorticoids in relation to the VDR.
Glucocorticoids (cortisol, cortisone) are excreted as part of a stress reaction (fight or flight, psychological stress) and ensure the supply of energy needed to solve the problem at hand. Within this context, the inhibition of the VDR appears logical, since it would ensure the shift of energy from the immune to motor systems. However, if the VDR is chronically inhibited, e.g., upon long-term cortisone treatment or chronic stress, this would explain the occurrence of pathological consequences such as osteoporosis. The clinically important immune-suppressive effect of glucocorticoids could be explained in part by the VDR blockade. Also the positive immune modulatory effect of low-dose cortisol treatment could be due to an influence on the VDR.
The literature contains an increasing number of data confirming this hypothesis. For example, chronically elevated glucocorticoid levels have been shown to compromise the effects of 1,25(OH)2D3 and promote the development of osteoporosis (49). Administration of 10 mg prednisolone, an artificial glucocorticoid, for seven days to healthy males resulted in enhanced renal calcium excretion, an elevation of biomarkers of bone degeneration and an increase in PTH levels. These effects could be compensated by the administration of 1,25(OH)2D3 (50). Another study showed that dexamethasone, another synthetic glucocorticoid, is able to potentiate the VDR-mediated transcription of CYP24A1 by a mechanism involving the recruitment of the glucocorticoid receptor to the CYP24A1 promoter region and its cooperation with C/EBPβ; since CYP24A1 catalizes the first step of 1,25(OH)2D3 degradation, dexamethasone effectively reduced 1,25(OH)2D3 concentrations (51). Finally, multiple putative glucocorticoid responsive elements could be identified within the VDR gene, which suggest a regulation of VDR expression through endogenous glucocorticoids (52). This is consistent with type II diabetics having both higher cortisol secretion (53) and lower VDR mRNA and protein levels (54) compared to healthy controls. In general, both inhibitory and activating tissue-specific effects on the VDR could be observed upon dexamethasone administration (52, 55).
Estrogens appear to positively stimulate vitamin D metabolism through a direct effect on the VDR (56, 57). An activation of the thyroid receptor, on the other hand, can have an inhibitory effect on vitamin D regulation via the VDR (58). These examples demonstrate that the response of the VDR can be modulated by a variety of physiological influences.
In addition, further pathophysiological mechanisms have emerged during the evolution of life. Due to the profound influence of vitamin D on the immune system it appears reasonable from an evolutionary standpoint to view the VDR as a strategically important target for pathological insults that aim to evade the immune system. For multiple tumor cell lines an anti-proliferative effect of 1,25(OH)2D3 has been described (34). An attenuation of these anti-proliferative effects would confer a growth advantage for tumor cells. Along these lines, colorectal cancer cells have been shown to regulate the expression or responsiveness of the VDR (59). A blockade of the VDR was also revealed as the pathophysiological mechanism exploited by osteosarcoma cells (60).
An increasing number of studies also describe the VDR as a strategic target of various pathogens. Lipopolysaccharides for example, which are sepsis inducing bacterial toxins, inhibit the expression of the VDR within THP-1 human monocytes (61).
In mammals, one of the central functions of the protein caspase-3 is the induction of programmed cell death (apoptosis). Thereby, it is possibly necessary to inhibit cellular vitamin D metabolism. Indeed, it was shown that caspase-3 is able to inhibit vitamin D metabolism by binding to and inactivating the VDR (62). Pathogens such as A. salmonicida, Legionella pneumophila, E. coli or Yersinia spp. in turn are able to interact with, or activate caspase-3 within cells, in this way inhibiting the VDR and attenuating the immune response of their host (62, 63).
Blockade of the VDR has also been described for other pathogens. A carefully conducted study has investigated the impact of Borrelia burgdorferi on the transcriptome of monocytes using whole genome microarrays [Supplementary Table S2 in (64)]. This technique revealed a 60-fold downregulation of the VDR by live Borrelia. In human B lymphocytes, it was shown that their infection with Epstein-Barr-virus (EBV) inhibits VDR mRNA and protein expression (65). Yenamandra et al. confirmed this VDR blockade through EBV in B lymphocytes (66) and later showed that the EBNA-3 protein is responsible for this blockade by its affinity to bind to the VDR (67). Finally, human cytomegalovirus induced a 88% inhibition of VDR expression in infected cells, leading to a gradual increase of the CYP27B1 gene to 970%; notably, no downregulation of the VDR was observed for influenza or adenovirus infection (68). The mechanisms how pathogens disrupt vitamin D signaling may provide a missing link between the association of infections and autoimmune pathogenesis. Indeed, pathogen infections have been discussed as triggers of autoimmune diseases for a longer time (69). For example, infections with campylobacter, EBV or influenza viruses have been causally connected to the development of Guillain-Barré syndrome (70). Attention has also been paid to the link between EBV infection and MS development (71). As described above, blockade of the VDR by EBV could thereby provide a plausible underlying mechanism behind this link.
In summary, all these examples demonstrate a more or less potent influence on the vitamin D system via the VDR by physiological and pathophysiological influences. In particular, the pathogen-mediated temporary or chronic blockade of the VDR can be explained from an evolutionary view in which pathogens have developed ways to attack this key target in order to downregulate their host's immune response. We thus propose the hypothesis that an interplay between acquired blockades of the VDR and polymorphisms affecting autoimmune disease susceptibility in either the VDR or other genes of the vitamin D system are able to cause a progressively severe form of low vitamin D responsiveness, which we refer to as acquired vitamin D resistance and which ultimately mediates the development of an autoimmune disease (Figure 2). Two other autoimmune disease-related factors fit nicely into this picture, namely a lack of sun exposure and the aging process, as intestinal vitamin D3 absorption (72), endogenous skin production (73) and hydroxylation (74) all have been shown to decrease with aging. Finally, environmental toxins could also be integrated into this hypothesis if they interfere with vitamin D metabolism. For example aluminum, which has been found in high concentrations in brain tissue from MS patients (75), was able to decrease renal CYP27B1 activity in the chick (76). Here, future research on the effects of environmental toxins such as aluminum and cadmium on the vitamin D system is urgently needed.
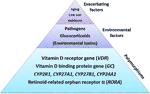
Figure 2 The etiology of acquired vitamin D resistance. Polymorphisms of genes within the vitamin D system constitute the basis for a susceptibitly towards developing vitamin D resistance, and hence autoimmune diseases. Partial blockades of the vitamin D receptor through pathogens, glucocorticoids (chronic stress) and – putatively – environmental toxins such as heavy metals may interact with such a susceptibility so that vitamin D resistance emerges. Finally, low sun exposure and aging, which correlate with autoimmune diseases as well, will exacerbate this situation further.
Discussion
Consequences of Vitamin D Resistance
The expression of the VDR as a gene regulatory protein in almost all tissues of the human body suggests functional roles well beyond those classically associated with calcium metabolism. Along these lines, vitamin D is more and more viewed as essential for maintaining physiological homeostasis. Vitamin D deficiency is associated with a plethora of cardiovascular and metabolic diseases including cancer, hypertension, infectious and autoimmune diseases (2, 7, 34). The proof of VDR expression in multiple cell types of the immune system such as CD4+/CD8+ lymphocytes, neutrophils and activated T-cell lymphocytes or antigen presenting cells (monocytes, macrophages or dendritic cells) underlines the importance of this receptor within this system (38). Mechanistically, multiple different effects of 1,25(OH)2D3 on the immune system have been described, including, but not limited to, chemotaxis, phagocytosis, proliferation, differentiation, cytokine production, antigen presentation, antibody, cathelicidin and hydrogen peroxide production and memory cell buildup (34). Only recently, Carlberg et al. identified a number of key genes of the immune system which are expressed by the VDR upon 1,25(OH)2D3 activation (48). Besides genes whose proteins play an essential role within the innate immune system by producing antimicrobial peptides, another group of proteins was identified which are closely connected to autoimmune processes. An example is the transmembrane protein Ninjurin1 (NINJ1) which is regulated by 1,25(OH)2D3. NINJ1 is involved in transmigration of antigen-presenting cells across the blood-brain barrier and in the pathogenesis of MS (77).
There also is an epigenetic influence of the VDR on genes of the immune system. An in vivo proof-of-principle study investigated chromatin accessibility in monocytes obtained from a human subject before and after a vitamin D bolus dose of 2000 µg (80,000 I.U.) (78). The bolus dose, which raised 25(OH)D3 levels by 7.6 ng/ml after two days, resulted in a significant opening or closing of hundreds of gene loci, the most prominent of which belonged to the human leucocyte antigen (HLA) system coded on chromosome 6. With the help of antigen-presenting proteins of the HLA system, the immune system is able to differentiate between endogenous and exogenous proteins. Therefore, a dysfunction within the HLA system predisposes for the development of autoimmune diseases. Polymorphisms of the HLA system for example constitute the most significant genetic influence on MS (79). Interestingly, a vitamin D responsive element could be identified at a gene within the HLA-DRB1 region, which is closely associated with the development of MS (80). Besides the epigenetic influence, this finding also underlines the transcriptional effect of vitamin D on the pathogenesis of this disease.
Looking beyond the box of autoimmune diseases to the mounting evidence of Vitamin D as a player in tumor development (81, 82), significant associations between SNPs of vitamin D system genes and common types of cancer (prostate, breast, colorectal and skin cancer) have been reported (83, 84), suggesting a similar predisposing role of SNPs for cancer as for autoimmune diseases. In these cases, a partial blockade of the VDR by pathogens could be another pathway within the established infectious etiology of cancer (85, 86).
In summary, there is much plausibility for acquired vitamin D resistance playing a pathological role in the development of autoimmune diseases, in this way providing an important component for our understanding and explanation of these diseases.
Therapy of Vitamin D Resistance
Currently no causal and therefore reliable therapies exist for correcting a blockade of the VDR. The only effective therapy to date is the high-dose vitamin D protocol, known after its inventor as the Coimbra protocol (30). With up to approximately 1000 I.U. vitamin D3 per kg body weight, the highest therapeutic vitamin D starting doses are thereby administered for the treatment of MS. For other autoimmune diseases much lower doses appear sufficient (Table 1). Hypercalcemia may be a major concern raised against this protocol. However, as discussed in more detail below, the vitamin D resistance appears to confer an intrinsic protection against hypercalcemia. In addition, the therapy requires the patient to take some precautionary measures. Besides the avoidance of milk products and a minimum fluid intake of 2.5 l/day, patients will need to consistently monitor multiple blood parameters and undergo regular sonographic checks of their kidneys. Practically, this requires close and regular contact with a certified Coimbra practitioner.

Table 1 Initial vitamin D3 doses used within the Coimbra protocol for the treatment of autoimmune diseases.
The Coimbra protocol follows a long medical tradition in which receptor resistances are compensated by applying higher doses. This is exemplified using a simplified analogy of insulin resistance therapy:
The current practice in type 2 diabetes management consists of calculating the required external insulin dose according to blood glucose levels. Thereby it is almost irrelevant how high the injected insulin dose actually is. It is the correction of the target parameter, i.e. blood glucose, which is pivotal. To achieve this goal, total daily insulin doses exceeding 100 I.U. can be utilized (87). These are doses that would normally be toxic in the absence of an underlying insulin resistance. The diabetic patient is to some extent protected against severe side effects of what would usually be considered an overdose, because this dose only acts physiologically – provided the applied doses are calculated correctly and there is close supervision by a physician.
Although we acknowledge that the causes of insulin resistance are very different from those of vitamin D resistance, and high-dose insulin injections cause comparatively more side effects than high-dose vitamin D3 injections, the above analogy illustrates the principles of the Coimbra protocol: The calculation of the required vitamin D dose depends on the grade of vitamin D resistance and the stage of the particular disease and incorporates the PTH concentration in analogy to the insulin/blood glucose example. Provided close supervision by an experienced physician, the applied high doses of vitamin D3 are causing neither hypercalcemia nor kidney damage. They will only exert physiological effects – in this way, the underlying vitamin D resistance acts protective against what would normally be considered a potentially toxic dose. In Supplementary Table 1 we provide data collected in the corresponding author's practice (DL) revealing no case of calcium excursion in a cohort of 41 patients observed over at least six months. The development of their PTH concentrations is plotted in Figure 3 and shows a significant decline at three months follow-up.
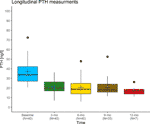
Figure 3 Longitudinal PTH measurements in a cohort of 41 relapsing-remitting multiple sclerosis patients treated by the corresponding author (DL) with the Coimbra protocol. Black lines indicate the median, blue crosses the mean. At three months follow-up, PTH levels had dropped significantly compared to the baseline measurement (Wilcoxon rank sum test, p<0.001), while there were no significant differences between any of the follow-up measurements. The reduction of PTH concentrations into the middle of the lower third of its laboratory-specific reference range is judged as an indicator for overcoming vitamin D resistance.
However, one case of a 39-year old symptomatic MS patient with hypercalcemia and renal insufficiency has been reported by a Swiss team of physicians (88). After seven months on the Coimbra protocol with 100,000 I.U. of vitamin D3 daily, his calcium concentration at hospital admission was 3.0 mmol/L, his 25(OH)D3 level 697 ng/ml (1742 nmol/l), and his PTH was 96 ng/l. The PTH level was thus much higher than typically observed on the Coimbra protocol (Figure 3). A possible explanation is that the patient was a carrier of the Multiple Endocrine Neoplasia, type 1 (MEN1) gene and had a history of elevated PTH levels already prior to starting the Coimbra protocol. Although this case highlights the possibility of high-dose vitamin D toxicity within the Coimbra protocol, it appears to be very rare. However, it also highlights the need for a diagnostic assessment of hyperparathyroidism prior to initiating the protocol as well as a regular endocrinological monitoring during therapy. It is noteworthy that the patient described in the case report wanted the authors to provide a brief account of his own standpoint according to which he and other patients he knew had improved their MS symptoms significantly on the protocol (88).
Such subjective improvements of autoimmune disease patients as well as safety data have also been collected within the network of Coimbra protocol physicians in Germany; these will be subject to future publications.
Finally, we would briefly like to mention the opinion held by some therapists that vitamin D administration is counterindicated in patients with vitamin D resistance. This hypothesis is inconsistent with the medical experience and understanding according to which resistances can be compensated with normal-to-high doses as described above. In particular, it is inconsistent with the experience gained by many Coimbra protocol physicians specialized in high dose vitamin D therapy that have collected many treatment successes thus far.
In summary, we have reviewed evidence for the hypothesis of an acquired form of vitamin D resistance, developing on the basis of a genetic susceptibility from certain SNPs within the vitamin D system and its interplay with chronic stress and/or pathogen infections that are able to partially block the VDR. Other factors that have been associated with autoimmune diseases such as low sun exposure, aging or environmental toxins could easily be integrated into this hypothesis since they would further exacerbate developing vitamin D resistance arising from the described mechanisms (Figure 2).
The hypothesis of acquired vitamin D resistance thus provides a plausible pathomechanism for the development of autoimmune diseases. We consider its therapeutic exploitation by high-dose vitamin D administration as a promising approach. Our key messages reflecting the knowledge about vitamin D resistance and its treatment are summarized in Table 2.

Table 2 Key points discussed in this paper.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
DL and RJK drafted the initial manuscript. DL and BS collected the data. FS and JS edited the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
DL and BS are certified Coimbra practitioners. BS owns the practice that apply the here described Coimbra protocol to patients with autoimmune diseases.
The remaining authors declare that the research was conducted in the absense of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fimmu.2021.655739/full#supplementary-material
References
1. Matsuoka LY, Wortsman J, Haddad JG, Hollis BW. In vivo threshold for cutaneous synthesis of vitamin D3. J Lab Clin Med (1989) 114:301–5.
Google Scholar
2. Gröber U, Spitz J, Reichrath J, Kisters K, Holick MF. Vitamin D: Update 2013. From rickets prophylaxis to general preventive healthcare. Dermatoendocrinol (2013) 5:331–47. doi: 10.4161/derm.26738
CrossRef Full Text | Google Scholar
3. Sawada N, Sakaki T, Ohta M, Inouye K. Metabolism of vitamin D3 by human CYP27A1. Biochem Biophys Res Commun (2000) 273:977–84. doi: 10.1006/bbrc.2000.3050
CrossRef Full Text | Google Scholar
4. Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res (2014) 55:13–31. doi: 10.1194/jlr.R031534
CrossRef Full Text | Google Scholar
5. Holick MF. Vitamin D Status: Measurement, Interpretation, and Clinical Application. Ann Epidemiol (2009) 19:73–8. doi: 10.1016/j.annepidem.2007.12.001
CrossRef Full Text | Google Scholar
6. Gröber U, Kisters K, Spitz J, Adamietz IA. Vitamin D in der onkologischen Intervention: Update 2015. Dtsch Z Für Onkol (2015) 47:173–7. doi: 10.1055/s-0035-1547572
CrossRef Full Text | Google Scholar
7. Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that vitamin d supplementation could reduce risk of influenza and covid-19 infections and deaths. Nutrients (2020) 12:988. doi: 10.3390/nu12040988
CrossRef Full Text | Google Scholar
8. Murdaca G, Tonacci A, Negrini S, Greco M, Borro M, Puppo F, et al. Emerging role of vitamin D in autoimmune diseases: An update on evidence and therapeutic implications. Autoimmun Rev (2019) 18:102350. doi: 10.1016/j.autrev.2019.102350
CrossRef Full Text | Google Scholar
9. Slominski AT, Kim T, Shehabi HZ, Semak I, Tang EKY, Nguyen MN, et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J (2012) 26:3901–15. doi: 10.1096/fj.12-208975
CrossRef Full Text | Google Scholar
10. Slominski AT, Kim TK, Shehabi HZ, Tang EKY, Benson HAE, Semak I, et al. In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol Cell Endocrinol (2014) 383:181–92. doi: 10.1016/j.mce.2013.12.012
CrossRef Full Text | Google Scholar
11. Slominski AT, Kim TK, Li W, Postlethwaite A, Tieu EW, Tang EKY, et al. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci Rep (2015) 5:14875. doi: 10.1038/srep14875
CrossRef Full Text | Google Scholar
12. Slominski AT, Li W, Kim TK, Semak I, Wang J, Zjawiony JK, et al. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol (2015) 151:25–37. doi: 10.1016/j.jsbmb.2014.11.010
CrossRef Full Text | Google Scholar
13. Slominski AT, Kim TK, Janjetovic Z, Brożyna AA, Żmijewski MA, Xu H, et al. Differential and overlapping effects of 20,23(OH)2 D3 and 1,25(OH)2 D3 on gene expression in human epidermal keratinocytes: Identification of AHR as an alternative receptor for 20,23(OH)2 D3. Int J Mol Sci (2018) 19:3072. doi: 10.3390/ijms19103072
CrossRef Full Text | Google Scholar
14. Slominski AT, Kim TK, Takeda Y, Janjetovic Z, Brozyna AA, Skobowiat C, et al. RORα and ROR γ are expressed in human skin and serve as receptors for endogenously produced noncalcemic 20-hydroxy- and 20,23-dihydroxyvitamin D. FASEB J (2014) 28:2775–89. doi: 10.1096/fj.13-242040
CrossRef Full Text | Google Scholar
15. Slominski AT, Kim TK, Hobrath JV, Oak ASW, Tang EKY, Tieu EW, et al. Endogenously produced nonclassical vitamin D hydroxy-metabolites act as "biased" agonists on VDR and inverse agonists on RORα and RORγ. J Steroid Biochem Mol Biol (2017) 173:42–56. doi: 10.1016/j.jsbmb.2016.09.024
CrossRef Full Text | Google Scholar
16. Slominski RM, Tuckey RC, Manna PR, Jetten AM, Postlethwaite A, Raman C, et al. Extra-adrenal glucocorticoid biosynthesis: implications for autoimmune and inflammatory disorders. Genes Immun (2020) 21:150–68. doi: 10.1038/s41435-020-0096-6
CrossRef Full Text | Google Scholar
17. Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, et al. T Helper 17 Lineage Differentiation Is Programmed by Orphan Nuclear Receptors RORα and RORγ. Immunity (2008) 28:29–39. doi: 10.1016/j.immuni.2007.11.016
CrossRef Full Text | Google Scholar
18. Peiser M. Role of Th17 cells in skin inflammation of allergic contact dermatits. Clin Dev Immunol (2013) 2013:261037. doi: 10.1155/2013/261037
CrossRef Full Text | Google Scholar
19. Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol (2008) 172:146–55. doi: 10.2353/ajpath.2008.070690
CrossRef Full Text | Google Scholar
20. Eftekharian MM, Noroozi R, Sayad A, Sarrafzadeh S, Toghi M, Azimi T, et al. RAR-related orphan receptor A (RORA): A new susceptibility gene for multiple sclerosis. J Neurol Sci (2016) 369:259–62. doi: 10.1016/j.jns.2016.08.045
CrossRef Full Text | Google Scholar
21. Carlberg C, Haq A. The concept of the personal vitamin D response index. J Steroid Biochem Mol Biol (2018) 175:12–7. doi: 10.1016/j.jsbmb.2016.12.011
CrossRef Full Text | Google Scholar
22. Saksa N, Neme A, Ryynänen J, Uusitupa M, De Mello VDF, Voutilainen S, et al. Dissecting high from low responders in a vitamin D3 intervention study. J Steroid Biochem Mol Biol (2015) 148:275–82. doi: 10.1016/j.jsbmb.2014.11.012
CrossRef Full Text | Google Scholar
23. Seuter S, Virtanen JK, Nurmi T, Pihlajamäki J, Mursu J, Voutilainen S, et al. Molecular evaluation of vitamin D responsiveness of healthy young adults. J Steroid Biochem Mol Biol (2017) 174:314–21. doi: 10.1016/j.jsbmb.2016.06.003
CrossRef Full Text | Google Scholar
24. Albright F, Butler AM, Bloomberg E. RICKETS RESISTANT TO VITAMIN D THERAPY. Am J Dis Child (1937) 54:529–47. doi: 10.1001/archpedi.1937.01980030073005
CrossRef Full Text | Google Scholar
25. Liberman UA, Eil C, Marx SJ. Resistance to 1,25-dihydroxyvitamin D. Association with heterogeneous defects in cultured skin fibroblasts. J Clin Invest (1983) 71:192–200. doi: 10.1172/JCI110759
CrossRef Full Text | Google Scholar
26. Malloy PJ, Eccleshall TR, Gross C, Van Maldergem L, Bouillon R, Feldman D. Hereditary vitamin D resistant rickets caused by a novel mutation in the vitamin D receptor that results in decreased affinity for hormone and cellular hyporesponsiveness. J Clin Invest (1997) 99:297–304. doi: 10.1172/JCI119158
CrossRef Full Text | Google Scholar
28. Schierbeck LL, Jensen TS, Bang U, Jensen G, Køber L, Jensen JEB. Parathyroid hormone and vitamin D—markers for cardiovascular and all cause mortality in heart failure. Eur J Heart Fail (2011) 13:626–32. doi: 10.1093/eurjhf/hfr016
CrossRef Full Text | Google Scholar
29. Bouillon R, Verstuyf A, Mathieu C, Van Cromphaut S, Masuyama R, Dehaes P, et al. Vitamin D resistance. Best Pract Res Clin Endocrinol Metab (2006) 20:627–45. doi: 10.1016/j.beem.2006.09.008
CrossRef Full Text | Google Scholar
30. Finamor DC, Sinigaglia-Coimbra R, Neves LCM, Gutierrez M, Silva JJ, Torres LD, et al. A pilot study assessing the effect of prolonged administration of high daily doses of vitamin D on the clinical course of vitiligo and psoriasis. Dermatoendocrinol (2013) 5:222–34. doi: 10.4161/derm.24808
CrossRef Full Text | Google Scholar
31. Ramos-Lopez E, Brück P, Jansen T, Herwig J, Badenhoop K. CYP2R1 (vitamin D 25-hydroxylase) gene is associated with susceptibility to type 1 diabetes and vitamin D levels in Germans. Diabetes Metab Res Rev (2007) 23:631–6. doi: 10.1002/dmrr.719
CrossRef Full Text | Google Scholar
32. Sundqvist E, Bäärnhielm M, Alfredsson L, Hillert J, Olsson T, Kockum I. Confirmation of association between multiple sclerosis and CYP27B1. Eur J Hum Genet (2010) 18:1349–52. doi: 10.1038/ejhg.2010.113
CrossRef Full Text | Google Scholar
33. Hallau J, Hamann L, Schumann RR, Worm M, Heine G. A promoter polymorphism of the vitamin D metabolism gene Cyp24a1 is associated with severe atopic dermatitis in adults. Acta Derm Venereol (2016) 96:169–72. doi: 10.2340/00015555-2226
CrossRef Full Text | Google Scholar
34. Bizzaro G, Antico A, Fortunato A, Bizzaro N. Vitamin D and autoimmune diseases: Is vitamin D receptor (VDR) polymorphism the culprit? Isr Med Assoc J (2017) 19:438–43.
Google Scholar
35. Mory DB, Gabbay MAL, Rocco ER, Kasamatsu T, Crispim F, Miranda WL, et al. High frequency of Vitamin D receptor gene polymorphism FokI in Brazilian Type 1 diabetes mellitus patients with clinical autoimmune thyroid disease. Diabetol Metab Syndr (2016) 8:29. doi: 10.1186/s13098-016-0145-5
CrossRef Full Text | Google Scholar
36. Gao XR, Yu YG. Meta-Analysis of the association between vitamin D receptor polymorphisms and the risk of autoimmune thyroid disease. Int J Endocrinol (2018) 2018:2846943. doi: 10.1155/2018/2846943
CrossRef Full Text | Google Scholar
37. Marini F, Falcini F, Stagi S, Fabbri S, Ciuffi S, Rigante D, et al. Study of vitamin D status and vitamin D receptor polymorphisms in a cohort of Italian patients with juvenile idiopathic arthritis. Sci Rep (2020) 10:17550. doi: 10.1038/s41598-020-74861-9
CrossRef Full Text | Google Scholar
38. Booth DR, Ding N, Parnell GP, Shahijanian F, Coulter S, Schibeci SD, et al. Cistromic and genetic evidence that the Vitamin D receptor mediates susceptibility to latitude-dependent autoimmune diseases. Genes Immun (2016) 17:213–9. doi: 10.1038/gene.2016.12
CrossRef Full Text | Google Scholar
39. Norman AW, Henry HL. Hormones. 3rd ed. London: Academic Press (2015).
Google Scholar
40. Racz A, Barsony J. Hormone-dependent translocation of vitamin D receptors is linked to transactivation. J Biol Chem (1999) 274:19352–60. doi: 10.1074/jbc.274.27.19352
CrossRef Full Text | Google Scholar
41. Carlberg C, Campbell MJ. Vitamin D receptor signaling mechanisms: Integrated actions of a well-defined transcription factor. Steroids (2013) 78:127–36. doi: 10.1016/j.steroids.2012.10.019
CrossRef Full Text | Google Scholar
43. Seuter S, Neme A, Carlberg C. Epigenome-wide effects of Vitamin D and their impact on the transcriptome of human monocytes involve CTCF. Nucleic Acids Res (2016) 44:4090–104. doi: 10.1093/nar/gkv1519
CrossRef Full Text | Google Scholar
44. Nurminen V, Neme A, Seuter S, Carlberg C. The impact of the vitamin D-modulated epigenome on VDR target gene regulation. Biochim Biophys Acta - Gene Regul Mech (2018) 1861:697–705. doi: 10.1016/j.bbagrm.2018.05.006
CrossRef Full Text | Google Scholar
45. Imani D, Razi B, Motallebnezhad M, Rezaei R. Association between vitamin D receptor (VDR) polymorphisms and the risk of multiple sclerosis (MS): An updated meta-analysis. BMC Neurol (2019) 19:339. doi: 10.1186/s12883-019-1577-y
CrossRef Full Text | Google Scholar
46. Tomei S, Singh P, Mathew R, Mattei V, Garand M, Alwakeel M, et al. The role of polymorphisms in vitamin D-related genes in response to vitamin D supplementation. Nutrients (2020) 12:2608. doi: 10.3390/nu12092608
CrossRef Full Text | Google Scholar
47. Valdivielso JM, Fernandez E. Vitamin D receptor polymorphisms and diseases. Clin Chim Acta (2006) 371:1–12. doi: 10.1016/j.cca.2006.02.016
CrossRef Full Text | Google Scholar
48. Koivisto O, Hanel A, Carlberg C. Key vitamin D target genes with functions in the immune system. Nutrients (2020) 12:1140. doi: 10.3390/nu12041140
CrossRef Full Text | Google Scholar
49. Mazziotti G, Formenti AM, Frara S, Doga M, Giustina A. Vitamin D and Glucocorticoid-Induced Osteoporosis. Front Horm Res (2017) 50:149–60. doi: 10.1159/000486078. Basel: Karger;.
CrossRef Full Text | Google Scholar
50. Gram J, Junker P, Nielsen HK, Bollerslev J. Effects of short-term treatment with prednisolone and calcitriol on bone and mineral metabolism in normal men. Bone (1998) 23:297–302. doi: 10.1016/S8756-3282(98)00097-0
CrossRef Full Text | Google Scholar
51. Dhawan P, Christakos S. Novel regulation of 25-hydroxyvitamin D3 24-hydroxylase (24(OH)ase) transcription by glucocorticoids: Cooperative effects of the glucocorticoid receptor, C/EBPβ, and the vitamin D receptor in 24(OH)ase transcription. J Cell Biochem (2010) 110:1314–23. doi: 10.1002/jcb.22645
CrossRef Full Text | Google Scholar
52. Hidalgo AA, Trump DL, Johnson CS. Glucocorticoid regulation of the vitamin D receptor. J Steroid Biochem Mol Biol (2010) 121:372–5. doi: 10.1016/j.jsbmb.2010.03.081
CrossRef Full Text | Google Scholar
53. Chiodini I, Adda G, Scillitani A, Coletti F, Morelli V, Di Lembo S, et al. Cortisol secretion in patients with type 2 diabetes: Relationship with chronic complications. Diabetes Care (2007) 30:83–8. doi: 10.2337/dc06-1267
CrossRef Full Text | Google Scholar
54. Yi B, Huang J, Zhang W, Li AM, Yang SK, Sun J, et al. Vitamin D receptor down-regulation is associated with severity of albuminuria in type 2 diabetes patients. J Clin Endocrinol Metab (2016) 101:4395–404. doi: 10.1210/jc.2016-1516
CrossRef Full Text | Google Scholar
55. Godschalk M, Levy JR, Downs RWJ. Glucocorticoids decrease vitamin D receptor number and gene expression in human osteosarcoma cells. J Bone Miner Res (1992) 7:21–7. doi: 10.1002/jbmr.5650070105
CrossRef Full Text | Google Scholar
56. Fleet JC, Schoch RD. Molecular mechanisms for regulation of intestinal calcium absorption by vitamin D and other factors. Crit Rev Clin Lab Sci (2010) 47:181–95. doi: 10.3109/10408363.2010.536429
CrossRef Full Text | Google Scholar
57. Gilad LA, Bresler T, Gnainsky J, Smirnoff P, Schwartz B. Regulation of vitamin D receptor expression via estrogen-induced activation of the ERK 1/2 signaling pathway in colon and breast cancer cells. J Endocrinol (2005) 185:577–92. doi: 10.1677/joe.1.05770
CrossRef Full Text | Google Scholar
58. Raval-Pandya M, Freedman LP, Li H, Christakos S. Thyroid Hormone Receptor Does Not Heterodimerize with the Vitamin D Receptor but Represses Vitamin D Receptor-Mediated Transactivation. Mol Endocrinol (1998) 12:1367–79. doi: 10.1210/mend.12.9.0165
CrossRef Full Text | Google Scholar
59. Ciulei G, Orasan OH, Coste SC, Cozma A, Negrean V, Procopciuc LM. Vitamin D and the insulin-like growth factor system: Implications for colorectal neoplasia. Eur J Clin Invest (2020) 50:e13265. doi: 10.1111/eci.13265
CrossRef Full Text | Google Scholar
60. Deng C, Ueda E, Chen KE, Bula C, Norman AW, Luben RA, et al. Prolactin blocks nuclear translocation of VDR by regulating its interaction with BRCA1 in osteosarcoma cells. Mol Endocrinol (2009) 23:226–36. doi: 10.1210/me.2008-0075
CrossRef Full Text | Google Scholar
61. Pramanik R, Asplin JR, Lindeman C, Favus MJ, Bai S, Coe FL. Lipopolysaccharide negatively modulates vitamin D action by down-regulating expression of vitamin D-induced VDR in human monocytic THP-1 cells. Cell Immunol (2004) 232:137–43. doi: 10.1016/j.cellimm.2005.03.004
CrossRef Full Text | Google Scholar
62. Malloy PJ, Feldman D. Inactivation of the human vitamin D receptor by caspase-3. Endocrinology (2009) 150:679–86. doi: 10.1210/en.2008-1217
CrossRef Full Text | Google Scholar
63. Wall DM, Mccormick BA. Bacterial secreted effectors and caspase-3 interactions. Cell Microbiol (2014) 16:1746–56. doi: 10.1111/cmi.12368
CrossRef Full Text | Google Scholar
64. Salazar JC, Duhnam-Ems S, La Vake C, Cruz AR, Moore MW, Caimano MJ, et al. Activation of human monocytes by live Borrelia burgdorferi generates TLR2-dependent and -independent responses which include induction of IFN-β. PloS Pathog (2009) 5:e1000444. doi: 10.1371/journal.ppat.1000444
CrossRef Full Text | Google Scholar
65. Morgan JW, Reddy GS, Uskokovic MR, May BK, Omdahl JL, Maizel AL, et al. Functional block for 1α,25-dihydroxyvitamin D3-mediated gene regulation in human B lymphocytes. J Biol Chem (1994) 269:13437–43. doi: 10.1016/S0021-9258(17)36851-5
CrossRef Full Text | Google Scholar
66. Yenamandra SP, Lundin A, Arulampalam V, Yurchenko M, Pettersson S, Klein G, et al. Expression profile of nuclear receptors upon Epstein - Barr virus induced B cell transformation. Exp Oncol (2009) 31:92–6.
Google Scholar
67. Yenamandra SP, Hellman U, Kempkes B, Darekar SD, Petermann S, Sculley T, et al. Epstein-Barr virus encoded EBNA-3 binds to vitamin D receptor and blocks activation of its target genes. Cell Mol Life Sci (2010) 67:4249–56. doi: 10.1007/s00018-010-0441-4
CrossRef Full Text | Google Scholar
68. Rieder FJJ, Gröschel C, Kastner MT, Kosulin K, Laengle J, Zadnikar R, et al. Human cytomegalovirus infection downregulates vitamin-D receptor in mammalian cells. J Steroid Biochem Mol Biol (2017) 165:356–62. doi: 10.1016/j.jsbmb.2016.08.002
CrossRef Full Text | Google Scholar
69. Getts DR, Chastain EML, Terry RL, Miller SD. Virus infection, antiviral immunity, and autoimmunity. Immunol Rev (2013) 255:197–209. doi: 10.1111/imr.12091
CrossRef Full Text | Google Scholar
70. Tam CC, O'Brien SJ, Petersen I, Islam A, Hayward A, Rodriguez LC. Guillain-Barré syndrome and preceding infection with Campylobacter, influenza and Epstein-Barr virus in the General Practice Research Database. PloS One (2007) 2:e344. doi: 10.1371/journal.pone.0000344
CrossRef Full Text | Google Scholar
71. Giovannoni G, Cutter GR, Lunemann J, Martin R, Münz C, Sriram S, et al. Infectious causes of multiple sclerosis. Lancet Neurol (2006) 5:887–94. doi: 10.1016/S1474-4422(06)70577-4
CrossRef Full Text | Google Scholar
72. Barragry JM, France MW, Corless D, Gupta SP, Switala S, Boucher BJ, et al. Intestinal cholecalciferol absorption in the elderly and in younger adults. Clin Sci Mol Med (1978) 55:213–20. doi: 10.1042/cs0550213
CrossRef Full Text | Google Scholar
73. Maclaughlin J, Holick MF, Kasper K. Aging Decreases the Capacity of Human Skin to Produce Vitamin D3. Nutr Clin Pract (1986) 1:57–8. doi: 10.1177/088453368600100118
CrossRef Full Text | Google Scholar
75. Mold M, Chmielecka A, Rodriguez MRR, Thom F, Linhart C, King A, et al. Aluminium in brain tissue in multiple sclerosis. Int J Environ Res Public Health (2018) 15:1777. doi: 10.3390/ijerph15081777
CrossRef Full Text | Google Scholar
76. Henry HL, Norman AW. Interactions Between Aluminum and the Actions and Metabolism of Vitamin D3 in the Chick. Calcif Tissue Int (1985) 37:484–90. doi: 10.1007/BF02557831
CrossRef Full Text | Google Scholar
77. Ifergan I, Kebir H, Terouz S, Alvarez JI, Lécuyer MA, Gendron S, et al. Role of Ninjurin-1 in the migration of myeloid cells to central nervous system inflammatory lesions. Ann Neurol (2011) 70:751–63. doi: 10.1002/ana.22519
CrossRef Full Text | Google Scholar
78. Carlberg C, Seuter S, Nurmi T, Tuomainen TP, Virtanen JK, Neme A. In vivo response of the human epigenome to vitamin D: A Proof-of-principle study. J Steroid Biochem Mol Biol (2018) 180:142–8. doi: 10.1016/j.jsbmb.2018.01.002
CrossRef Full Text | Google Scholar
79. Lemke D. Die Bedeutung von Lebensstilfaktoren für die Sekundärprävention der Multiplen Sklerose. [Medical thesis]. Germany: University of Mainz (2019).
Google Scholar
80. Ramagopalan SV, Maugeri NJ, Handunnetthi L, Lincoln MR, Orton SM, Dyment DA, et al. Expression of the multiple sclerosis-associated MHC class II allele HLA-DRB1*1501 is regulated by vitamin D. PloS Genet (2009) 5:e1000369. doi: 10.1371/journal.pgen.1000369
CrossRef Full Text | Google Scholar
81. González-Sancho JM, Larriba MJ, Muñoz A. Wnt and vitamin D at the crossroads in solid cancer. Cancers (Basel) (2020) 12:3434. doi: 10.3390/cancers12113434
CrossRef Full Text | Google Scholar
82. Fernández-Barral A, Bustamante-Madrid P, Ferrer-Mayorga G, Barbáchano A, Larriba MJ, Muñoz A. Vitamin D effects on cell differentiation and stemness in cancer. Cancers (Basel) (2020) 12:2413. doi: 10.3390/cancers12092413
CrossRef Full Text | Google Scholar
83. Vidigal VM, Silva TD, de Oliveira J, Pimenta CAM, Felipe AV, Forones NM. Genetic polymorphisms of vitamin D receptor (VDR), CYP27B1 and CYP24A1 genes and the risk of colorectal cancer. Int J Biol Markers (2017) 32:e224–30. doi: 10.5301/jbm.5000248
CrossRef Full Text | Google Scholar
84. Gnagnarella P, Raimondi S, Aristarco V, Johansson HA, Bellerba F, Corso F, et al. Vitamin D Receptor Polymorphisms and Cancer. Adv Exp Med Biol (2020) 1268:53–114. doi: 10.1007/978-3-030-46227-7_4
CrossRef Full Text | Google Scholar
85. Zur Hausen H. Condylomata acuminata and human genital cancer. Cancer Res (1976) 36:1–2.
Google Scholar
86. zur Hausen H. Infections Causing Human Cancer. 1st ed. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co (2006).
Google Scholar
87. Kedia R, Desouza C, Smith LM, Shivaswamy V. A retrospective review of insulin requirements in patients using U-500 insulin hospitalized to a Veterans Affairs Hospital. J Diabetes Complications (2017) 31:874–9. doi: 10.1016/j.jdiacomp.2017.02.008
CrossRef Full Text | Google Scholar
88. Lutz N, Steffen P, Nigg L. Nebenwirkung einer nicht evidenzbasierten MS-Therapie. Swiss Med Forum - Schweizerisches Medizin-Forum (2020) 20:230–3. doi: 10.4414/smf.2020.08365
CrossRef Full Text | Google Scholar
High Dose Vitamin C Autoimmune
Source: https://www.frontiersin.org/articles/655739



0 Komentar